Share this post
Antioxidants and reproductive health
We live in an increasingly toxic world, with literally thousands of different potential toxins being released into our water, air, and soil each day by industry, agricultural practices, and our daily living.[1],[2],[3],[4] They cycle into our food supply, accumulating in animals and plants.[5],[6],[7],[8] Many environmental pollutants do not degrade over time, and thus continue to accumulate; these chemicals are classified as persistent organic pollutants (POPs). Pollutants such as these also don’t remain in one place – they are cycled in our environment and distribute globally.[9],[10]
The best way to address extensive exposure to environmental toxins is to work with a healthcare practitioner who is familiar with protocols to facilitate their removal prior to attempts at conception.
Environmental toxicants not only impact our longevity and risk for chronic disease,[11],[12],[13] they also gravely affect reproductive health.[14] Our exposure to toxic pollutants is not only a reproductive factor in our older years, it has an impact when we are young as well.[15],[16] Because females have a limited number of gametes, set from the moment they are born, and their detoxification surfaces and pathways have a lower capacity then men, the long-term impact of toxicants on female fertility may be more dramatic.[17]
The two key ways in which environmental toxins adversely affect our health is by their endocrine effects and the oxidative damage they cause. Although the best way to address extensive exposure to toxins such as these is to work with a healthcare practitioner who is familiar with protocols to facilitate their removal prior to attempts at conception, support with antioxidant therapies also can be of help for many. Herein, we take a look at the evidence behind melatonin and CoQ10 as antioxidants that support female reproductive health.
Melatonin
Multiple studies suggest melatonin has positive effects on oocyte quality and fertilization rates, as well as the common female conditions of endometriosis and polycystic ovarian syndrome.
It may come as a surprise that the hormone antioxidant best known for the role it plays in our sleep cycle has also been studied for its reproductive impact. Extensive research exists on the anti-inflammatory and immune-regulating effects of melatonin,[18] which also may give rise to its positive effects on both female and male fertility.[19],[20]
The study of melatonin’s impact on female fertility largely grew out of research in the late 80’s which found that high levels of melatonin exist in female preovulatory follicular fluid – approximately 3.5 times higher than the corresponding serum concentration.[21] Animal and human studies suggest that the melatonin found in the follicular fluid is taken up the ovaries from systemic circulation, although some may also be produced in the ovaries themselves.[20] Melatonin has been shown to impact follicular growth, with significantly higher levels of endogenous melatonin being found in the larger, healthier follicles versus the small follicles that were obtained from women for fertilization procedures.[22]
The majority of research concerning melatonin for female fertility is in women undergoing IVF-embryo transfer (ET) procedures, using their own harvested oocytes. Supplementation with 3 mg of melatonin/day was found to lead to a higher percentage of mature oocytes being retrieved for IVF-ET procedure, with trends to a higher clinical pregnancy rate compared to placebo.[23] In another study, melatonin taken at 3 mg/day was shown to significantly improve fertilization rates in women who failed to become pregnant in their last IVF-ET procedure.[24] Multiple additional human studies also point to positive effects on oocyte quality and fertilization rates.[25],[26],[27],[28]
The effects of melatonin on female reproductive health are not limited to those undergoing IVF treatment of course – the potential benefits of this antioxidant/hormone also extend to several other conditions that affect the female reproductive system. Numerous cellular and animal studies suggest melatonin may have a positive impact on endometriosis, recurrent spontaneous abortion, polycystic ovarian syndrome, and immune, chemotherapy, or radiation-induced primary ovarian insufficiency,[29] as well as the normal fertility decline with aging.[30],[31]
Evidence also points to melatonin as a supplement that can ameliorate oxidative stress and perhaps improve male fertility in the setting of natural conception as well as IVF procedures.[32],[33]
CoQ10
Human studies suggest CoQ10 may positively impact outcomes in women with diminished ovarian reserve or polycystic ovarian syndrome who were going through fertility treatments.
In similar ways, CoQ10 also helps protect the gametes of both men and women. Like melatonin, CoQ10 is a fat-soluble antioxidant, and helps protect cellular membranes from oxidative stress. Additionally, CoQ10 is an essential nutrient for mitochondrial energy production – which is important for both male and female reproductive health. Because CoQ10 levels decline with aging, it perhaps is not a surprise that a primary focus of research with this nutrient concerns diseases and conditions associated with aging: cardiovascular and metabolic disease,[34] neurodegenerative disorders,[35] sarcopenia and muscle strength,[36] skin changes such as wrinkles,[37] and “reproductive aging”, the term used to describe non-specific fertility decline with age.
Alterations in mitochondrial energy production are one factor that contributes to reproductive aging and the decline of viable oocytes with age.[38] The primary energy-generating pathway in the developing oocyte takes place in the mitochondria and depends on adequate amounts of CoQ10.[39] In an aged animal model, dietary supplementation with CoQ10 was shown to significantly increase the number of ovulated eggs, while other mitochondrial nutrients (lipoic acid and resveratrol) were not observed to have such an effect.[40]
In studies of female mice with compromised antioxidant defense pathways, supplementation with CoQ10 also helped prevent the related decline in fertility.[41] In cell studies, CoQ10 has additionally been shown to help protect germ cells (a precursor to gametes) against oxidative damage due to exposure to BPA.[42]
In women taking CoQ10, significant improvements were seen in the number of retrieved oocytes, the quality of embryos, and the fertilization rate.
Clinical studies with CoQ10 have shown promising results. In one of these studies, women (< 35 years of age) who had diminished ovarian reserve were randomized to receive placebo or 200 mg of CoQ10 three times daily for 60 days prior to IVF-ET treatment.[43] In women taking CoQ10, significant improvements were seen in the number of retrieved oocytes, the quality of embryos, and the fertilization rate. While the ET procedure was canceled due to poor quality in 22.89% of women receiving the placebo, it was only canceled in 8.33% of those taking CoQ10. Clinical pregnancy and live birth rates after ET both tended to be higher in those given CoQ10, but the difference was not significant compared to placebo.
CoQ10 also has been shown to improve multiple aspects of fertility in women with polycystic ovarian syndrome (PCOS), a condition commonly associated with fertility struggles. Treatment with clomiphene citrate is the first-line therapy to induce ovulation in females with PCOS who are attempting to become pregnant, however, oftentimes, it is unsuccessful.
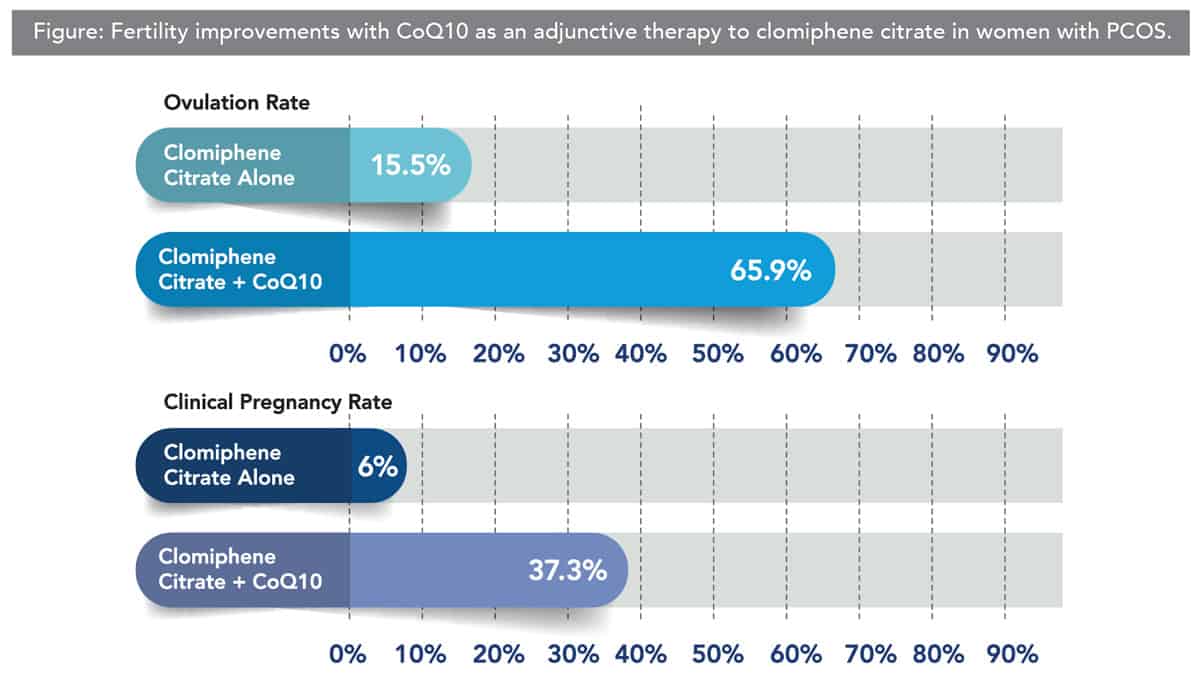
Clearly, the positive findings of the clinical and preclinical studies suggest CoQ10 may be of great benefit for women with a diminished fertility due to a variety of causes, including the general decline with aging. However, an important point was brought up in a comprehensive review of the studies considering the impact CoQ10 has on reproductive aging.[45] The point made was that many of the anti-aging fertility benefits seen in mouse models are with dietary supplementation of CoQ10 for a period that would be equivalent to a decade in human years, suggesting this antioxidant should be part of the daily protocol for any woman who wishes to consider childbirth later in life.
Fertility challenges can be very difficult for all involved parties. Although our modern era offers numerous medical tools that we can turn to, it is important to remember the nutritional basics. The antioxidant supplements discussed herein, the nutrients discussed in Part 1, and additional supplements that have been researched in the setting of male reproductive health, should offer further hope to the individuals dealing with these challenges.
Click here to see References[1] Soto AM, Sonnenschein C. Environmental causes of cancer: endocrine disruptors as carcinogens. Nat Rev Endocrinol. 2010 Jul;6(7):363-70.
[2] Lelieveld J, et al. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015 Sep 17;525(7569):367-71.
[3] Klecka G, et al. Chemicals of emerging concern in the Great Lakes Basin: an analysis of environmental exposures. Rev Environ Contam Toxicol. 2010;207:1-93.
[4] Manzetti S, et al. Chemical properties, environmental fate, and degradation of seven classes of pollutants. Chem Res Toxicol. 2014 May 19;27(5):713-37.
[5] Sabir SM, et al. Effect of environmental pollution on quality of meat in district Bagh, Azad Kashmir. Pakistan J Nutr. 2003;2(2):98-101.
[6] Poliakova OV, et al. Accumulation of persistent organic pollutants in the food chain of Lake Baikal. Toxicol & Enviro Chem. 2000 Apr 1;75(3-4):235-43.
[7] Benavides MP, et al. Cadmium toxicity in plants. Brazil J Plant Physiol. 2005 Mar;17(1):21-34.
[8] de Geus HJ, et al. Environmental occurrence, analysis, and toxicology of toxaphene compounds. Environ Health Perspect. 1999 Feb;107 Suppl 1(Suppl 1):115-44.
[9] Wania F, Mackay D. Peer reviewed: tracking the distribution of persistent organic pollutants. Environ Sci Technol. 1996 Aug 27;30(9):390A-6A.
[10] Lovett GM, et al. Effects of air pollution on ecosystems and biological diversity in the eastern United States. United States. Ann N Y Acad Sci. 2009 Apr;1162(1):99-135.
[11] Carpenter DO. Environmental contaminants as risk factors for developing diabetes. Rev Environ Health. 2008 Jan-Mar;23(1):59-74.
[12] Irigaray P, et al. Lifestyle-related factors and environmental agents causing cancer: an overview. Biomed Pharmacother. 2007 Dec;61(10):640-58.
[13] Bhatnagar A, et al. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res. 2006 Sep 29;99(7):692-705.
[14] Mendiola J, et al. Exposure to environmental toxins in males seeking infertility treatment: a case-controlled study. Reprod Biomed Online. 2008 Jun;16(6):842-50.
[15] Perry MJ, et al. A prospective study of serum DDT and progesterone and estrogen levels across the menstrual cycle in nulliparous women of reproductive age. Am J Epidemiol. 2006 Dec 1;164(11):1056-64.
[16] Windham GC, et al. Exposure to organochlorine compounds and effects on ovarian function. Epidemiology. 2005 Mar;16(2):182-90.
[17] Butter ME. Are women more vulnerable to environmental pollution? J Human Ecol. 2006 Nov 1;20(3):221-6.
[18]FOCUS Newsletter. Melatonin, immune function, and respiratory health. Fall 2020 Special Immunity Issue. Pages 14-18.
[19] Frungieri MB, et al. Local actions of melatonin in somatic cells of the testis. Int J Mol Sci. 2017 May 31;18(6):1170.
[20] Tamura H, et al. Melatonin and the ovary: physiological and pathophysiological implications. Fertil Steril. 2009 Jul;92(1):328-43.
[21] Brzezinski A, et al. Melatonin in human preovulatory follicular fluid. J Clin Endocrinol Metab. 1987 Apr;64(4):865-7.
[22] Nakamura Y, et al. Increased endogenous level of melatonin in preovulatory human follicles does not directly influence progesterone production. Fertil Steril. 2003 Oct;80(4):1012-6.
[23] Batıoğlu AS, et al. The efficacy of melatonin administration on oocyte quality. Gynecol Endocrinol. 2012 Feb;28(2):91-3.
[24] Tamura H, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008 Apr;44(3):280-7.
[25] Pacchiarotti A, et al. Effect of myo-inositol and melatonin versus myo-inositol, in a randomized controlled trial, for improving in vitro fertilization of patients with polycystic ovarian syndrome. Gynecol Endocrinol. 2016;32(1):69-73.
[26] Nishihara T, et al. Oral melatonin supplementation improves oocyte and embryo quality in women undergoing in vitro fertilization-embryo transfer. Gynecol Endocrinol. 2014 May;30(5):359-62.
[27] Eryilmaz OG, et al. Melatonin improves the oocyte and the embryo in IVF patients with sleep disturbances, but does not improve the sleeping problems. J Assist Reprod Genet. 2011 Sep;28(9):815-20.
[28] Unfer V, et al. Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: a prospective, longitudinal, cohort study. Gynecol Endocrinol. 2011 Nov;27(11):857-61.
[29] Yang HL, et al. Pleiotropic roles of melatonin in endometriosis, recurrent spontaneous abortion, and polycystic ovary syndrome. Am J Reprod Immunol. 2018 Jul;80(1):e12839.
[30] Tamura H, et al. Long-term melatonin treatment delays ovarian aging. J Pineal Res. 2017 Mar;62(2).
[31] Zhang L, et al. Melatonin regulates the activities of ovary and delays the fertility decline in female animals via MT1/AMPK pathway. J Pineal Res. 2019 Apr;66(3):e12550.
[32] Frungieri MB, et al. Local actions of melatonin in somatic cells of the testis. Int J Mol Sci. 2017 May 31;18(6):1170.
[33] Riviere E, et al. Melatonin daily oral supplementation attenuates inflammation and oxidative stress in testes of men with altered spermatogenesis of unknown aetiology. Mol Cell Endocrinol. 2020 Sep 15;515:110889.
[34] Díaz-Casado ME, et al. The paradox of coenzyme Q10 in aging. Nutrients. 2019 Sep 14;11(9):2221.
[35] Hernández-Camacho JD, et al. Coenzyme Q10 supplementation in aging and disease. Front Physiol. 2018 Feb 5;9:44.
[36] Fischer A, et al. Coenzyme Q10 status as a determinant of muscular strength in two independent cohorts. PLoS One. 2016 Dec 1;11(12):e0167124.
[37] Žmitek K, et al. The effect of dietary intake of coenzyme Q10 on skin parameters and condition: results of a randomised, placebo-controlled, double-blind study. Biofactors. 2017 Jan 2;43(1):132-40.
[38] Roth Z. Symposium review: Reduction in oocyte developmental competence by stress is associated with alterations in mitochondrial function. J Dairy Sci. 2018 Apr;101(4):3642-54.
[39] Bentov Y, Casper RF. The aging oocyte–can mitochondrial function be improved? Fertil Steril. 2013 Jan;99(1):18-22.
[40] Burstein E, et al. Co-enzyme Q10 supplementation improves ovarian response and mitochondrial function in aged mice. Fertility and Sterility. 2009 Sep 1;92(3):S31.
[41] Ishii N, et al. Ascorbic acid and CoQ10 ameliorate the reproductive ability of superoxide dismutase 1-deficient female mice. Biol Reprod. 2020 Feb 12;102(1):102-15.
[42] Hornos Carneiro MF, et al. Antioxidant CoQ10 restores fertility by rescuing bisphenol A-induced oxidative DNA damage in the Caenorhabditis elegans germline. Genetics. 2020 Feb;214(2):381-95.
[43] Xu Y, et al. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: a randomized controlled trial. Reprod Biol Endocrinol. 2018 Mar 27;16(1):29.
[44] Balen AH, Rutherford AJ. Managing anovulatory infertility and polycystic ovary syndrome. BMJ. 2007 Sep 29;335(7621):663-6.
[45] Ben-Meir A, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015 Oct;14(5):887-95.
The information provided is for educational purposes only. Consult your physician or healthcare provider if you have specific questions before instituting any changes in your daily lifestyle including changes in diet, exercise, and supplement use.
Share this post
Dr. Carrie Decker
Related posts
Glandulars: A Key Therapy in Resolving Fatigue?
Restorative therapies from the animal kingdom for advanced thyroid and adrenal support The many causes of fatigue There’s usually no one “magic bullet” cure for fatigue. This is likely because there is usually no one consistent cause. Fatigue can arise from disrupted sleep patterns, infections, blood sugar imbalances, nutritional deficiencies, allergies, or air pollution….
Don’t Forget to Water Your Kids!
Keeping hydration up in the dog-days of summer and first weeks of back-to-school Kids. Water. Fruits. Vegetables. In this series of words, which does not belong? Of course, we could evaluate them by various forms of reasoning (i.e. kids contain a high level of water) but in their natural form we probably don’t see…
CoQ10 vs Astaxanthin
Two Powerhouse Nutrients for Optimal Athletic Performance When it comes to introducing these two powerhouse nutrients, most people have heard of coenzyme Q10 (CoQ10). It has a great reputation as a powerful antioxidant and has been well researched as a cardioprotective nutrient.[1],[2] It has been shown to have a positive impact on blood lipid…
Healthy Mouth, Healthy Body
Why we shouldn’t ignore the oral microbiome If you’re like me, you’d prefer not to dwell on the millions of bacteria living in your mouth. But if you care about your health, you can’t ignore the oral microbiome! Up to 700 different species of bacteria live in various niches within the mouth.[1],[2],[3] Even individuals…
Breaking Bad: Homocysteine and Aging
The role of B vitamins in cardiovascular and neurological health “If I’d known I was going to live this long, I’d have taken better care of myself.” – Eubie Blake, American composer, upon reaching the age of 100 By now it’s widely known that high blood cholesterol levels can be harmful to our health….
Melatonin: Is It Really the Sedative We Think It Is?
Numerous clinical studies suggest otherwise, a look at their findings not related to sleep Since its discovery in 1958,[1] we have learned much about the diverse functions of melatonin in animals, where it serves not only as a circadian rhythm regulator, but also as an antioxidant, immunoregulatory and anti-inflammatory molecule, hormone, metal chelator, and…
Categories
- Botanicals (56)
- GI Health (53)
- Healthy Aging (121)
- Immune Support (39)
- In The News (39)
- Kids Health (21)
- Stress and Relaxation (50)
- Uncategorized (1)
- Video (9)
- Vitamins & Minerals (51)




