Share this post
An essential nutrient for detoxification and the brain
At least nine trace elements are essential for humans: iron, zinc, copper, manganese, iodine, chromium, selenium, cobalt, and molybdenum.[1] Molybdenum has been studied far less than minerals such as iodine, zinc, or selenium. Nevertheless, our understanding of the importance of molybdenum has grown exponentially in recent years.[2]
As a supplement, molybdenum has long been used to treat copper toxicity, and in particular the inherited disorder known as Wilson’s disease in which copper accumulates in tissues. The role of molybdenum in balancing copper levels suggests that it may also be useful as metabolic support for cancer patients, as discussed below.
In humans and animals, four different enzymes requiring molybdenum have been identified that play specialized roles in the body, including the detoxification of sulfites and purines from dietary and metabolic sources.[3] Studies of genetic diseases that influence these pathways are yielding numerous insights into the importance of molybdenum for human health, as we’ll discuss here.
Molybdenum for copper toxicity
Molybdenum, copper, zinc, and other minerals found in food and water can interact with each other, so an excess of one mineral can produce a relative deficiency of another. For example, both molybdenum and zinc can interfere with copper absorption.[4],[5]
The ability of molybdenum to antagonize copper is an advantage in cases of copper toxicity, which can occur due to environmental or industrial exposure.
The ability of molybdenum to antagonize copper is an advantage in cases of copper toxicity, which can occur due to environmental or industrial exposure. Copper overload can lead to hepatitis (inflammation of the liver), kidney disease, and neurological disorders.[6],[7] A form of molybdenum known as tetrathiomolybdate, which is classified as a drug, has been shown to chelate (bind) excess copper and thereby reduce copper levels in the body.[8],[9],[10]
In humans, the copper-lowering ability of tetrathiomolybdate has been used to treat Wilson’s disease, an inherited disorder in which harmful levels of copper accumulate in the liver, brain, eyes (leading to Kayser-Fleischer rings around the iris), and other organs.[11] If left untreated, the excess copper can cause liver disease and neurological dysfunction.[12] Tetrathiomolybdate is one agent that is used to help relieve the toxicity.[4],[13],[14],[15]
Tetrathiomolybdate is also being investigated as an inhibitor of the angiogenesis (new blood vessel formation) that is seen in solid cancers. The rationale is that copper is required for blood vessel formation within tumors, a process that is necessary for cancer metastasis. Scientists have thus reasoned that lowering copper might inhibit cancer progression.[16] In support of this concept, several phase II clinical trials have shown promising results with tetrathiomolybdate for individuals with advanced kidney cancer,[17] prostate cancer,[18] and breast cancer.[19]
Scientists also have discovered that a familial form of amyotrophic lateral sclerosis (ALS, also known as Lou Gehrig’s disease) can be caused by a mutation in an enzyme known as copper-zinc superoxide dismutase (SOD1). The mutation in SOD1 is associated with focal accumulations of copper in the brain and spinal cord, which contribute to the disease symptoms.[20],[21],[22] This discovery prompted scientists to study the effects of copper binding therapies, including tetrathiomolybdate, in a mouse model of familial ALS. The results showed that tetrathiomolybdate not only delayed the disease onset and progression, but also prolonged survival.[23] Human clinical studies are needed to confirm these findings.
Unlike tetrathiomolybdate, the forms of molybdenum present in foods and dietary supplements do not chelate copper directly. These more natural forms of molybdenum, such as ammonium molybdate and molybdenum glycinate, appear to reduce absorption and increase the excretion of copper in the urine.[24],[25] Taken together, the evidence suggests that supplemental molybdenum may be helpful for conditions associated with copper accumulation.
Molybdenum as an enzyme cofactor
Molybdenum is essential for the activity of four human enzymes with specialized roles in detoxification, including sulfite oxidase and xanthine oxidase (discussed below).[12]
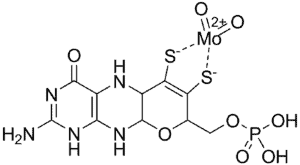
The molybdenum cofactor (MoCo) for these enzymes is comprised of molybdenum bound to a substance known as molybdopterin, which is synthesized within the body in a process involving several steps.[3] The final active cofactor is shown below.
Molybdenum cofactor (MoCo). Image: Wikimedia Commons
Rare inherited diseases have been identified in which molybdopterin synthesis is impaired, resulting in a deficiency of MoCo.[26] Infants born with this condition typically suffer from seizures, intellectual disability, and other abnormalities that are fatal if not treated. Proper diagnosis by a pediatric neurologist and subsequent supplementation with the missing molybdopterin precursor can ameliorate the condition in some cases.[27],[28]
Detoxification of sulfites
Molybdenum supplementation may help to relieve the symptoms of sulfite sensitivity because it helps the body eliminate it.
Our bodies are continuously exposed to sulfites, which are rapidly degraded under normal conditions in order to prevent toxicity.
Sulfite comes from both internal and external sources. For example, sulfite is continuously generated within the body from the breakdown of sulfur-containing amino acids such as methionine and cysteine, which are abundant in foods.[29]
Many foods and beverages contain free sulfites, including wine, beer, dried fruits, molasses, and many processed foods.[30] Sulfite sensitivity can occur when too much sulfite is taken in to the body or it isn’t able to be properly metabolized. Approximately 3% to 10% of adult asthmatics may be sensitive to sulfite additives, with symptoms ranging from migraines and bronchoconstriction to life-threatening anaphylactic reactions.[31] For this reason, sulfites are among the potential allergens that have to be labeled on food and drink products.[32] A sulfite sensitive individual would also poorly tolerate supplements such as N-acetylcysteine, which contains the offending sulfur molecule.
Sulfites also are produced by the metabolism of sulfur dioxide (SO2), an inhalable air pollutant that can trigger asthma attacks in some individuals.[33],[34] Inhaled SO2 is metabolized to bisulfite and sulfite, which have been shown to cause bronchoconstriction.[35],[36]
Under normal conditions, the body converts sulfite to sulfate, rendering it non-toxic. This step is carried out by sulfite oxidase (SO), a molybdenum-dependent enzyme.[37],[38] Dietary molybdenum deficiency is associated with reduced SO activity, as shown in animal models.[39] Thus, molybdenum supplementation may help to relieve the symptoms of sulfite sensitivity because it helps the body eliminate it.
A rare genetic disease, in which SO is completely missing, is known as inherited sulfite oxidase deficiency (ISOD).[40],[41] MoCo deficiency causes a syndrome that is very similar to ISOD.[42],[43] In both of these conditions, the body is unable to detoxify sulfites. Excessive levels of sulfites and other sulfated compounds accumulate in the brain, lungs, liver, and other organs.[26],[40],[42] Novel therapies are being investigated for these rare disorders.
Detoxification of xanthine
Xanthine is a molecule derived from the breakdown of purine nucleotides, which are produced by the breakdown of DNA and RNA. Molybdenum-dependent enzymes (xanthine oxidase and aldehyde oxidase) are necessary to break down xanthine to uric acid, which is excreted in the urine.[12],[44] Owing to genetic variations in xanthine oxidase (XO), the rate of xanthine metabolism and uric acid excretion varies from low to high within the population.[45]
In addition to converting purine nucleotides to uric acid, XO is necessary for the metabolism of thiopurine drugs. Such drugs are used to treat leukemia and inflammatory bowel disease, and to prevent transplant rejection.[46],[47] Insufficient XO activity can reduce thiopurine metabolism and produce serious side effects in individuals taking such medications.[48] Due to the critical nature of these medications for the conditions they treat, the possible use of molybdenum supplementation in excess of the typical dietary intake should be managed by the prescribing physician.
Genetic variants in an enzyme involved in the production of molybdenum cofactor have been discovered in some individuals with autism spectrum disorder and may increase the risk of the condition.
In children with hereditary defects of XO or MoCo, xanthine builds up in the urine and often causes kidney stones.[44],[49],[50] The buildup of purines is also toxic for the brain, and high xanthine levels are associated with mental delay and autistic behavior in these syndromes.[50],[51]
Recently, genetic changes in an enzyme in the MoCo pathway have been discovered in some individuals with autism spectrum disorder (ASD).[52],[53] These genetic variants impair the production of MoCo, and significantly increase the risk of ASD when they are present.[53] This discovery greatly adds to our understanding of ASD and creates new opportunities for the diagnosis and treatment of some individuals with this disorder.
Detoxification of mutagens and TMAO
A molybdenum-dependent enzyme known as mARC helps break down certain harmful nucleoside derivatives that can otherwise cause DNA damage. If these compounds are not removed from the body, they may be incorporated into DNA, potentially contributing to cancerous cellular mutations.[54],[55]
mARC also helps metabolize trimethylamine oxide (TMAO),[56] a bacterial metabolite produced in the gut that is associated with heart and Alzheimer’s disease.[57],[58] These findings suggest a possible role for mARC in disease prevention.
Dietary molybdenum deficiency
Severe molybdenum deficiency is rare in humans, although it has been documented in a patient receiving total parenteral nutrition without molybdenum supplementation.[59]
Although severe deficiencies may be rare, studies of molybdenum-deficient animals suggests that subclinical molybdenum deficiency may be fairly common, even in humans.[60]
By way of background, molybdenum deficiency has been seen in sheep grazing on a type of plant that is low in molybdenum and high in purines.[61] Such animals are susceptible to motor neuron disease (MND), a neurodegenerative condition that is characterized by muscle weakness, muscle atrophy, and breathing difficulties.[61],[62]
Interestingly, certain features of MND in animals are similar to those of amyotrophic lateral sclerosis (ALS, also known as Lou Gehrig’s disease) in humans.[60] This raises the possibility that low molybdenum levels might contribute to the development of ALS. According to this theory, insufficient molybdenum intake could increase one’s susceptibility to the neurotoxic effects of dietary purines and sulfites. Low uric acid levels and increased sulfite levels have been observed in patients with ALS (the non-familial type), which further supports this hypothesis.[63],[64]
Patients with Parkinson’s disease (PD) also have been shown to have elevated levels of sulfur-containing amino acid levels and depressed uric acid levels,[65],[66] consistent with suboptimal molybdenum nutrition.[67] Further studies are needed to assess whether molybdenum supplementation might help reduce the risk or progression of either PD or ALS over the long term.
Molybdenum requirements
Molybdenum is required in very small amounts in the diet.[68] The RDA for molybdenum is 45 mcg per day, however, the tolerable upper limit is 2,000 mcg per day.[68] Foods that are a good source of molybdenum include lamb, beef liver, pork, lentils, peas, and molasses.[12]
Individuals with sulfite sensitivity or with abnormally low uric acid levels, suggestive of low sulfite oxidase or xanthine oxidase activity, respectively, should consult a qualified health professional to determine whether molybdenum supplementation may be beneficial.
Click here to see References[1] Mehri A. Trace elements in human nutrition (II) – an update. Int J Prev Med. 2020 Jan 3;11:2.
[2] Wuebbens MM, et al. Insights into molybdenum cofactor deficiency provided by the crystal structure of the molybdenum cofactor biosynthesis protein MoaC. Structure. 2000 Jul 15;8(7):709-18.
[3] Mendel RR. The molybdenum cofactor. J Biol Chem. 2013 May 10; 288(19): 13165-72.
[4] Alvarez HM, et al. Tetrathiomolybdate inhibits copper trafficking proteins through metal cluster formation. Science. 2010 Jan 15;327(5963):331-4.
[5] Osredkar J, Sustar N. Copper and zinc, biological role and significant of copper/zinc imbalance. J Clinic Toxicol. 2011;S3:001.
[6] Daniel KG, et al. Copper storage diseases: Menkes, Wilsons, and cancer. Front Biosci. 2004 Sep 1;9:2652-62.
[7] Hsu HW, et al. Environmental and dietary exposure to copper and its cellular mechanisms linking to Alzheimer’s disease. Toxicol Sci. 2018 Jun 1;163(2):338-45.
[8] Seifert M, et al. The biological and toxicological importance of molybdenum in the environment and in the nutrition of plants, animals and man: Part V: Essentiality and toxicity of molybdenum. Acta Alimentaria. 2010;39(1):12-26.
[9] Kumaratilake JS, Howell JM. Effects of intravenously administered tetra-thiomolybdate on the distribution of copper in the liver and kidney of copper loaded sheep: a histochemical study. Res Vet Sci. 1987 Mar;42(2):154-61.
[10] Ogra Y, Suzuki KT. Targeting of tetrathiomolybdate on the copper accumulating in the liver of LEC rats. J Inorg Biochem. 1998 Apr;70(1):49-55.
[11] Członkowska A. Wilson disease. Nat Rev Dis Primers. 2018 Sep 6;4(1):21.
[12] Novotvy JA, Peterson CA. Molybdenum. Adv Nutr. 2018;9(3):272-3.
[13] Suttle NF. Copper imbalances in ruminants and humans: unexpected common ground. Adv Nutr. 2012 Sep 1;3(5):666-74.
[14] Brewer GJ, et al. Treatment of Wilson’s disease with tetrathiomolybdate: V. Control of free copper by tetrathiomolybdate and a comparison with trientine. Transl Res. 2009 Aug;154(2):70-7.
[15] Weiss KH, et al. Bis-choline tetrathiomolybdate in patients with Wilson’s disease: an open-label, multicentre, phase 2 study. Lancet Gastroenterol Hepatol. 2017 Dec;2(12):869-76.
[16] Wu D, et al. A novel mitochondrial amidoxime reducing component 2 is a favorable indicator of cancer and suppresses the progression of hepatocellular carcinoma by regulating the expression of p27. Oncogene. 2020 Aug 18:1-4.
[17] Redman BG, et al. Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer. Clin Cancer Res. 2003 May;9(5):1666-72.
[18] Henry NL, et al. Phase II trial of copper depletion with tetrathiomolybdate as an antiangiogenesis strategy in patients with hormone-refractory prostate cancer. Oncology. 2006;71(3-4):168-75.
[19] Chan N, et al. Influencing the tumor microenvironment: a phase II study of copper depletion using tetrathiomolybdate in patients with breast cancer at high risk for recurrence and in preclinical models of lung metastases. Clin Cancer Res. 2017 Feb 1;23(3):666-76.
[20] Johnston JA, et al. Formation of high molecular weight complexes of mutant Cu, Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12571-6.
[21] Li QX, et al. Overexpression of Abeta is associated with acceleration of onset of motor impairment and superoxide dismutase 1 aggregation in an amyotrophic lateral sclerosis mouse model. Aging Cell. 2006 Apr;5(2):153-65.
[22] Tokuda E, et al. Dysregulation of intracellular copper homeostasis is common to transgenic mice expressing human mutant superoxide dismutase-1s regardless of their copper-binding abilities. Neurobiol Dis. 2013 Jun;54:308-19.
[23] Tokuda E, et al. Ammonium tetrathiomolybdate delays onset, prolongs survival, and slows progression of disease in a mouse model for amyotrophic lateral sclerosis. Exp Neurol. 2008 Sep;213(1):122-8.
[24] Vyskočil A, Viau C. Assessment of molybdenum toxicity in humans. J Appl Toxicol. 1999 May;19(3):185-92.
[25] Deosthale YG, Gopalan C. The effect of molybdenum levels in sorghum (Sorghum vulgare Pers.) on uric acid and copper excretion in man. Br J Nutr. 1974 May;31(3):351-5.
[26] Atwal PS, Scaglia F. Molybdenum cofactor deficiency. Mol Genet Metab. 2016 Jan;117(1):1-4.
[27] Veldman A, et al. Successful treatment of molybdenum cofactor deficiency type A with cPMP. Pediatrics. 2010 May;125(5):e1249-54.
[28] Hitzert MM, et al. Favorable outcome in a newborn with molybdenum cofactor type A deficiency treated with cPMP. Pediatrics. 2012 Oct;130(4):e1005-10.
[29] Kohl JB, et al. Homeostatic impact of sulfite and hydrogen sulfide on cysteine catabolism. British Journal of Pharmacology. 2019 Feb;176(4):554-70.
[30] Amit SK, et al. A review on mechanisms and commercial aspects of food preservation and processing. Agriculture and Food Security. 2017 Dec 1;6(1):51.
[31] Vally H, Misso NLA. Adverse reactions to the sulphite additives. Gastroenterol Hepatol Bed Bench. Winter 2012;5(1):16-23.
[32] University of Nebraska, Institute of Agriculture and Natural Resources. Sulfites – USA [Internet]. Lincoln (NE): University of Nebraska; 2020 [cited 2020 Sep 2]. Available from: https://farrp.unl.edu/sulfites-usa
[33] Reno AL, et al. Mechanisms of heightened airway sensitivity and responses to inhaled SO2 in asthmatics. Environ Health Insights. 2015 Apr 1;9(Suppl 1):13-25.
[34] Gunnison AF, et al. Distribution, metabolism and toxicity of inhaled sulfur dioxide and endogenously generated sulfite in the respiratory tract of normal and sulfite oxidase-deficient rats. J Toxicol Environ Health. 1987;21(1-2):141-62.
[35] Fine JM, et al. The roles of pH and ionic species in sulfur dioxide- and sulfite-induced bronchoconstriction. Am Rev Respir Dis. 1987 Nov;136(5):1122-6.
[36] Song A, et al. Bisulfite and sulfite as derivatives of sulfur dioxide alters biomechanical behaviors of airway smooth muscle cells in culture. Inhal Toxicol. 2014 Feb;26(3):166-74.
[37] Schwarz G, Belaidi AA. Molybdenum in human health and disease. Met Ions Life Sci. 2013;13:415-50.
[38] Cohen HJ, et al. Molecular basis of the biological function of molybdenum. The relationship between sulfite oxidase and the acute toxicity of bisulfite and SO2. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3655-9.
[39] Yoshida M, et al. Low molybdenum state induced by tungsten as a model of molybdenum deficiency in rats. Biol Trace Elem Res. 2015 May;165(1):75-80.
[40] Misko AL, et al. Delineating the phenotypic spectrum of sulfite oxidase and molybdenum cofactor deficiency. Neurol Genet. 2020 Jul 14;6(4):e486.
[41] Feng C, et al. Sulfite oxidizing enzymes. Biochim Biophys Acta. 2007 May;1774(5):527-39.
[42] Carmi-Nawi N, et al. Prenatal brain disruption in molybdenum cofactor deficiency. J Child Neurol. 2011 Apr;26(4):460-4.
[43] Kumar A, et al. S-sulfocysteine/NMDA receptor-dependent signaling underlies neurodegeneration in molybdenum cofactor deficiency. J Clin Invest. 2017 Dec 1;127(12):4365-78.
[44] Ichida K, et al. Mutations associated with functional disorder of xanthine oxidoreductase and hereditary xanthinuria in humans. Int J Mol Sci. 2012 Nov 21;13(11):15475-95.
[45] Kudo M, et al. Functional characterization of human xanthine oxidase allelic variants. Pharmacogenet Genomics. 2008 Mar;18(3):243-51.
[46] Fotoohi AK, et al. Thiopurines: factors influencing toxicity and response. Biochem Pharmacol. 2010 May 1;79(9):1211-20.
[47] Lim SZ, Chua EW. Revisiting the role of thiopurines in inflammatory bowel disease through pharmacogenomics and use of novel methods for therapeutic drug monitoring. Front Pharmacol. 2018 Oct 8;9:1107.
[48] Stiburkova B, et al. Thiopurine-induced toxicity is associated with dysfunction variant of the human molybdenum cofactor sulfurase gene (xanthinuria type II). Toxicol Appl Pharmacol. 2018 Aug 15;353:102-8.
[49] Bradbury MG, et al. Acute renal failure due to xanthine stones. Pediatr Nephrol. 1995 Aug;9(4):476-7.
[50] Zannolli R, et al. Hereditary xanthinuria type II associated with mental delay, autism, cortical renal cysts, nephrocalcinosis, osteopenia, and hair and teeth defects. J Med Genet. 2003 Nov;40(11):e121.
[51] Sebesta I, et al. Hereditary xanthinuria is not so rare disorder of purine metabolism. Nucleosides Nucleotides Nucleic Acids. 2018;37(6):324-8.
[52] Féron F, et al. Olfactory stem cells reveal MOCOS as a new player in autism spectrum disorders. Mol Psychiatry. 2016 Sep;21(9):1215-24.
[53] Taheri M, et al. The rs594445 in MOCOS gene is associated with risk of autism spectrum disorder. Metab Brain Dis. 2020 Mar;35(3):497-501.
[54] Tejada-Jimenez M, et al. From the eukaryotic molybdenum cofactor biosynthesis to the moonlighting enzyme mARC. Molecules. 2018 Dec 11;23(12):3287.
[55] Plitzko B, et al. The pivotal role of the mitochondrial amidoxime reducing component 2 in protecting human cells against apoptotic effects of the base analog N6-hydroxylaminopurine. J Biol Chem. 2015 Apr 17;290(16):10126-35.
[56] Schneider J, et al. Detoxification of trimethylamine n-oxide by the mitochondrial amidoxime reducing component mARC. Chem Res Toxicol. 2018 Jun 18;31(6):447-53.
[57] Fu BC, et al. Associations of plasma trimethylamine N-oxide, choline, carnitine, and betaine with inflammatory and cardiometabolic risk biomarkers and the fecal microbiome in the Multiethnic Cohort Adiposity Phenotype Study. Am J Clin Nutr. 2020 Jun 1;111(6):1226-34.
[58] Vogt NM, et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimers Res Ther. 2018 Dec 22;10(1):124.
[59] Abumrad NN, et al. Amino acid intolerance during prolonged total parenteral nutrition reversed by molybdate therapy. Am J Clin Nutr. 1981;34(11):2551-9.
[60] Bourke CA. Molybdenum deficiency produces motor nervous effects that are consistent with amyotrophic lateral sclerosis. Front Neurol. 2016 Mar 8;7:28.
[61] Bourke CA. Motor neurone disease in molybdenum-deficient sheep fed the endogenous purine xanthosine: possible mechanism for Tribulus staggers. Aust Vet J. 2012 Jul;90(7):272-4.
[62] Bourke CA. Molybdenum deprivation, purine ingestion and an astrocyte‐associated motor neurone syndrome in sheep: assumed clinical effects of inosine. Aust Vet J. 2015 Mar;93(3):79-83.
[63] Keizman D, et al. Low uric acid levels in serum of patients with ALS: further evidence for oxidative stress? J Neurol Sci. 2009 Oct 15;285(1-2):95-9.
[64] Woolsey PBE. Cysteine, sulfite, and glutamate toxicity: a cause of ALS? J Altern Complement Med. 2008 Nov;14(9):1159-64.
[65] Heafield MT, et al. Plasma cysteine and sulphate levels in patients with motor neurone, Parkinson’s and Alzheimer’s disease. Neurosci Lett. 1990 Mar 2;110(1-2):216-20.
[66] Weisskopf MG, et al. Plasma urate and risk of Parkinson’s disease. Am. J. Epidemiol. 2007;166:561-7.
[67] Bourke CA. Astrocyte dysfunction following molybdenum-associated purine loading could initiate Parkinson’s disease with dementia. NPJ Parkinsons Dis. 2018 Mar 20;4:7.
[68] National Institutes of Health. Molybdenum: Fact Sheet for Health Professionals. Bethesda (MD): U S Department of Health and Human Services; 2020 [cited 2020 Sep 2]. Available from: https://ods.od.nih.gov/factsheets/Molybdenum-HealthProfessional/
The information provided is for educational purposes only. Consult your physician or healthcare provider if you have specific questions before instituting any changes in your daily lifestyle including changes in diet, exercise, and supplement use.
Share this post
Marina MacDonald, MS, PhD
Related posts
Study Identifies Widespread Inadequacies of Immune Health Nutrients
Key nutrient shortfalls may increase the risk of infections Ideally, a well-balanced, nutrient-dense diet, including a variety of colorful fruits and vegetables, whole grains, legumes, healthy fats, and a balanced selection of proteins can meet all of one’s daily requirements for essential vitamins and minerals, as well as essential fatty acids (EFAs), amino acids,…
Breaking Bad: Homocysteine and Aging
The role of B vitamins in cardiovascular and neurological health “If I’d known I was going to live this long, I’d have taken better care of myself.” – Eubie Blake, American composer, upon reaching the age of 100 By now it’s widely known that high blood cholesterol levels can be harmful to our health….
Vitamin D for Allergies
The data behind vitamin D for asthma, eczema, and atopic conditions More than 50 million Americans have an allergy of some kind. If you or a family member has allergies, you know how irritating they can be (literally). Allergies occur when the immune system overreacts to one or more allergens by producing antibodies called…
The Fat-Soluble Vitamins: A, D, E and K
Nutrients for strong bones and a healthy heart Vitamins are classified into two groups: fat-soluble (vitamins A, D, E, and K) and water-soluble (B-complex vitamins and vitamin C). Fat-soluble vitamins are absorbed best when consumed with higher-fat foods.[1] Fatty fish, vegetable oils, green vegetables, and nuts and seeds are good sources of vitamins A,…
How Vitamin C Can Kick Addiction
The versatile vitamin’s mechanisms of action This article serves as Part 3 in our series on vitamin C, pain, and opioid use disorder. Check out Part 1 to learn about the vitamin’s use in the management of pain. In Part 2 we dive into its potential for easing opioid withdrawal symptoms and drug cravings….
A Better Diet for Better Sperm Health
Simple ways to improve male fertility If you’re planning to start a family, here’s how to improve your chances of success. About 2/3 of couples succeed in getting pregnant within six months of starting unprotected sex. However, as many as 1 in 6 couples do not become pregnant within a year. Although the focus…
Categories
- Botanicals (56)
- GI Health (53)
- Healthy Aging (121)
- Immune Support (39)
- In The News (39)
- Kids Health (21)
- Stress and Relaxation (50)
- Uncategorized (1)
- Video (9)
- Vitamins & Minerals (51)